Which Of The Following Statements About Gluconeogenesis In Animal Cells Is True?
Introduction
Gluconeogenesis refers to a group of metabolic reactions, some of them highly exergonic and irreversible, which are regulated both locally and globally (by insulin, glucagon, and cortisol). The purpose of this organisation, localized in both the cytosol and mitochondria, is to maintain blood glucose level abiding throughout fasting land. The remainder betwixt stimulatory and inhibitory hormones regulates the charge per unit of gluconeogenesis. Liver and secondarily the kidney are the organs that supply circulating blood and consequently, diverse tissues with glucose. Many tissues depend primarily on glucose to maintain adequate energy levels for their proper function during fasting.
Fundamentals
Several tissues, including the brain, erythrocytes, renal medulla, the lens and cornea of the eye, testes, skeletal muscles during do, require continuous glucose supply. Amid these tissues, the brain uses glucose exclusively in both fed state and fasting state except for prolonged fasting, which uses ketones. Notably, the daily corporeality of glucose used by the brain accounts for 70% of the total glucose produced by the liver in a normal fasting person.[1]
Initially, during the starting time hours of fasting, hepatic glycogenolysis is the primary source of glucose. Glucose stored every bit glycogen can embrace the energy needs roughly for i twenty-four hours; the amount of glucose supplied by glycogen reserves is 190 g while the daily needs for glucose are 160 one thousand. After several hours of starvation, gluconeogenesis and glycogenolysis contribute as to blood glucose. The amount of glucose supplied by glycogen decreases speedily while the increase in the glucose fraction contributed by gluconeogenesis results in keeping constant the total amount of glucose produced. Estimates are that 54% of glucose comes from gluconeogenesis after fourteen hours of starvation, and this contribution raises to 64% after 22 hours and up to 84% after 42 hours.[ii] Withal, hours later that glycogen stores deplete, the body uses every bit glucose sources lactate, glycerol, glucogenic amino acids, and odd chain fatty acids. In prolonged fasting, kidney participation in gluconeogenesis is increased and is responsible for about 40% of total gluconeogenesis.[3]
Alanine, produced in skeletal muscles by protein catabolism and subsequent transamination reactions, is shuttled out in blood and taken up by the liver. Inside hepatocytes, alanine undergoes transamination into pyruvate, used for gluconeogenesis. Glucose produced in the liver is shuttled out in circulation and taken upwards by muscle cells for use in ATP production (Cahill bicycle). Other gluconeogenic amino acids (east.g., methionine, histidine, valine) every bit well as gluconeogenic and ketogenic (e.g., phenylalanine, isoleucine, threonine, tryptophane) get transaminated into unlike intermediates of the gluconeogenic pathway.[4]
In red blood cells and other tissues (lens) that lack mitochondria besides equally the exercising musculus tissue that favors anaerobic metabolism, glucose is converted to pyruvate and afterward to lactate. Lactate is secreted into plasma and picked up by the liver for conversion into glucose (Cori cycle) via a redox reaction catalyzed by lactate dehydrogenase.[v]
Fat acids are stored equally triglycerides and mobilized by the hormone-sensitive lipase (HSL); glycerol from the triglyceride construction is released in blood to be taken up past the liver, phosphorylated by glycerol kinase and oxidized into dihydroxyacetone phosphate -an intermediate of gluconeogenesis/ glycolysis pathway- by glycerol phosphate dehydrogenase. Odd-concatenation fatty acids, in dissimilarity to the ketogenic even- chain fatty acids, are converted with beta-oxidation into propionyl CoA. The latter converts after several steps into methylmalonyl CoA. Methylmalonyl CoA mutase/B12 catalyzes the conversion of the latter into succinyl-CoA. Succinyl-CoA is an intermediate of TCA cycle that is eventually converted into oxaloacetic acid and enters equally such the gluconeogenesis pathway. Even-chain fatty acids and purely ketogenic amino acids (leucine, lysine) that convert to acetyl-CoA cannot enter gluconeogenesis as no pathway tin can reverse the step catalyzed by pyruvate dehydrogenase (PDH).[half-dozen]
Information technology is worth mentioning that in sure conditions, such as ischemic strokes and brain tumor development, astrocytes have increased activity of gluconeogenic enzymes, and they use as substrates lactate, alanine, aspartate, glutamate.[7]
Regulation Overview
I) Glucagon regulates gluconeogenesis through:
-
Changes in allosteric regulators (reduces the levels of fructose-ii,6 bisphosphate)
-
Covalent modification of enzyme activeness (phosphorylation of pyruvate kinase results in its inactivation)
-
Induction of enzymes factor expression (glucagon via CRE response elements increases the expression of PEPCK
II) Acetyl CoA activates pyruvate carboxylase allosterically
III) Substrate availability might increase or decrease the rate of gluconeogenesis
4) AMP inhibits fructose-1,6 bisphosphatase allosterically
For gluconeogenesis to occur, the ADP/ATP ratio must be very low, since gluconeogenesis is an free energy demanding process requiring high energy molecules to be spent in several steps. In between meals, during early fasting, when prison cell via TCA wheel has generated sufficient ATP levels, the increased ATP levels inhibit several highly regulated TCA cycle enzymes (citrate synthase, isocitrate dehydrogenase, a-ketoglutarate dehydrogenase). Acetyl-CoA is the indicator of cells metabolic activity and functions as a gluconeogenesis regulator at a local level. Acetyl-CoA levels back up and allosterically activate pyruvate carboxylase. In this way, the cell makes sure that gluconeogenesis and TCA cycle will non happen simultaneously.
Mechanism
Pyruvate generation from phosphoenolpyruvate is the last irreversible pace of gluconeogenesis. Once cells are committed into the gluconeogenesis pathway, the reverse reaction occurs in two steps to go around the irreversible step and synthesize phosphoenolpyruvate from pyruvate.
1) The starting time step involves pyruvate carboxylase (PC), a ligase, adding a carboxyl grouping on pyruvate to create oxaloacetate. The enzyme consumes one ATP molecule, uses equally a cofactor biotin (vitamin B7) and uses a CO2 molecule as a source of carbon. Biotin is bound to a lysine remainder of PC. Afterwards ATP hydrolysis, an intermediate molecule PC-biotin-CO2 forms, that carboxylates pyruvate forming oxaloacetate. This reaction, autonomously from forming an intermediate for gluconeogenesis, provides oxaloacetic acid to TCA wheel (anaplerotic reaction).[viii] In muscle cells, PC is used mainly for anaplerotic reasons. The enzyme also requires magnesium. Pyruvate carboxylation happens in mitochondria; then via malate shuttle, oxaloacetate is being shuttled into the cytosol to be phosphorylated. Malate can cantankerous the inner mitochondrial membrane while oxaloacetic acid cannot. In cytosol along with the oxidation of oxaloacetic acid into malate, NAD+ gets reduced into NADH. The produced NADH is used in a subsequent pace when 1,3 bisphosphoglycerate converts into glyceraldehyde-3 phosphate.[9]
ii) The next exergonic reaction catalyzed by PEP carboxykinase (PEPCK), a lyase, uses GTP equally a phosphate donor to phosphorylate oxaloacetate and form PEP. Glucocorticoids induce PEPCK gene expression; cortisol subsequently bounden its steroid receptor intracellularly moves within the cell nucleus and binds with its zinc finger domain, the glucocorticoid response chemical element (GRE) on DNA.[10]
three) The residual of the reactions are reversible and common with gluconeogenesis. Enolase, a lyase, cleaves carbon-oxygen bonds and catalyzes the conversion of PEP into two-phosphoglycerate. Phosphoglycerate mutase, an isomerase, catalyzes the conversion of two-phosphoglycerate to iii-phosphoglycerate by transferring a phosphate from carbon-2 to carbon-3. Phosphoglycerate kinase using ATP as a phosphate donor and Mg+2 to stabilize with its positive charge the phosphotransfer reaction converts 3-phosphoglycerate to one,iii- bisphosphoglycerate. Glyceraldehyde 3-phosphate dehydrogenase catalyzes the reduction of 1,3-bisphosphoglycerate to glyceraldehyde 3-phosphate. NADH is oxidized every bit it donates its electrons for the reaction. Equally described earlier, glycerol phosphate from triglyceride catabolism is converted somewhen into DHAP. Triosephosphate isomerase converts DHAP into glyceraldehyde iii-phosphate. Aldolase A converts glyceraldehyde iii-phosphate into fructose-1,vi bisphosphate.[eleven]
4) The post-obit irreversible footstep involves the conversion of fructose 1,half-dozen bisphosphate into fructose-vi phosphate. This step is important as it is the rate-limiting stride of gluconeogenesis. Fructose-1,6 bisphosphatase catalyzes the dephosphorylation of fructose-1,half dozen bisphosphate, requiring bivalent metal cations (Mg+2, Mn+two); this is a highly regulated stride both globally and locally. Locally, increased ATP levels, too as increased levels of citrate (the showtime intermediate of TCA cycle), activate the enzyme, while increased AMP and increased fructose-ii,half dozen bisphosphate (F2,6BP) inactivate the enzyme. Glucagon by binding to its receptor, a GPCR, activates adenylate cyclase. The resulting increase in cyclic AMP (army camp) levels leads to the activation of protein kinase A (PKA). PKA phosphorylates fructose 2,six bisphosphatase (F2,6BPase) and phosphofructokinase-two (PFK-two). Phosphorylated PFK-2 is inactive while F2,6BPase is active and catalyzes the dephosphorylation of fructose ii,6 bisphosphate. Dephosphorylated F-two,6BP is inactive; hence, information technology does not have whatever negative event on F1,6BPase.[12][13]
5) The last irreversible reaction involves glucose-6 phosphatase catalyzing the hydrolysis of glucose-6 phosphate into glucose. This enzyme is expressed primarily in liver as well as in kidneys and intestinal epithelium. The reaction happens in the endoplasmic reticulum of the cells. Muscle cells do not express glucose-6 phosphatase every bit they produce glucose to maintain their own free energy needs.[14]
Clinical Significance
Von Gierke Illness- Glycogen storage disease type 1
Liver cells lack glucose-half dozen phosphatase, the enzyme required to release glucose from liver cells by dephosphorylating them. Von Gierke disease is a condition affecting both glycogenolysis and gluconeogenesis since the missing enzyme is common in both pathways resulting in accumulation of glucose-6 phosphate in liver cells. Symptoms include:
-
Hepatomegaly and kidney enlargement due to glycogen accumulation
-
Astringent fasting hypoglycemia since liver cells cannot release glucose in blood postprandially
-
Lactic acidosis since accumulated glucose-6 phosphate blocks gluconeogenesis and consequently lactate uptake
-
Hypertriglyceridemia, since increased levels of glucose-6 phosphate favor glycolysis and acetyl-CoA production, leading to increased malonyl-CoA synthesis and subsequent inhibition of carnitine acyltransferase i (the rate-limiting mitochondrial enzyme of fatty acid beta-oxidation);
Hyperuricemia is the issue of increased uric acid production (glc-6P that via HMP shunt is converted into ribose-5P and purines) and decreased uric acid excretion (uric acid competes with lactate for excretion via the same organic acid transporter in proximal renal tubules).[15][xvi] Other symptoms include protruding abdomen (hepatomegaly), truncal obesity and short height,[17] muscle wasting too equally a rounded doll'due south confront.[fifteen]
Pyruvate Carboxylase deficiency
Pyruvate carboxylase deficiency is a condition where cells lack pyruvate carboxylase or have an altered enzyme and manifest with lactic acidosis, hyperammonemia, and hypoglycemia. Hyperammonemia is due to pyruvate non being converted into oxaloacetic acid. Oxaloacetic acid gets transaminated into aspartate; reduction in aspartate levels results in the reduced introduction of ammonia into the urea cycle.[18]
Review Questions
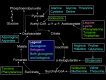
Figure
Gluconeogenesis. Image courtesy Dr Chaigasame
References
- i.
-
SCHEINBERG P. OBSERVATIONS ON CEREBRAL CARBOHYDRATE METABOLISM IN Human being. Ann Intern Med. 1965 Feb;62:367-71. [PubMed: 14259220]
- 2.
-
Chandramouli Five, Ekberg K, Schumann WC, Kalhan SC, Wahren J, Landau BR. Quantifying gluconeogenesis during fasting. Am J Physiol. 1997 December;273(6):E1209-15. [PubMed: 9435538]
- iii.
-
Gerich JE, Meyer C, Woerle HJ, Stumvoll M. Renal gluconeogenesis: its importance in man glucose homeostasis. Diabetes Intendance. 2001 Feb;24(two):382-91. [PubMed: 11213896]
- four.
-
Felig P. The glucose-alanine wheel. Metabolism. 1973 Feb;22(two):179-207. [PubMed: 4567003]
- 5.
-
Draoui Northward, Feron O. Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis Model Mech. 2011 November;4(half dozen):727-32. [PMC gratuitous article: PMC3209642] [PubMed: 22065843]
- 6.
-
Ferrannini Eastward, Barrett EJ, Bevilacqua Due south, DeFronzo RA. Effect of fat acids on glucose production and utilization in man. J Clin Invest. 1983 Nov;72(five):1737-47. [PMC free article: PMC370462] [PubMed: 6138367]
- 7.
-
Yip J, Geng X, Shen J, Ding Y. Cerebral Gluconeogenesis and Diseases. Front Pharmacol. 2016;7:521. [PMC free article: PMC5209353] [PubMed: 28101056]
- viii.
-
Adina-Zada A, Zeczycki TN, Attwood PV. Regulation of the structure and activity of pyruvate carboxylase by acetyl CoA. Arch Biochem Biophys. 2012 Mar 15;519(2):118-thirty. [PMC free article: PMC3293938] [PubMed: 22120519]
- 9.
-
Jitrapakdee S, St Maurice Grand, Rayment I, Cleland WW, Wallace JC, Attwood PV. Structure, machinery and regulation of pyruvate carboxylase. Biochem J. 2008 Aug 01;413(iii):369-87. [PMC free article: PMC2859305] [PubMed: 18613815]
- 10.
-
Stark R, Guebre-Egziabher F, Zhao X, Feriod C, Dong J, Alves TC, Ioja South, Pongratz RL, Bhanot Due south, Roden G, Cline GW, Shulman GI, Kibbey RG. A role for mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-Chiliad) in the regulation of hepatic gluconeogenesis. J Biol Chem. 2014 Mar 14;289(11):7257-63. [PMC free article: PMC3953244] [PubMed: 24497630]
- 11.
-
Chung ST, Chacko SK, Sunehag AL, Haymond MW. Measurements of Gluconeogenesis and Glycogenolysis: A Methodological Review. Diabetes. 2015 Dec;64(12):3996-4010. [PMC free article: PMC4657587] [PubMed: 26604176]
- 12.
-
Hers HG. The control of glycolysis and gluconeogenesis by poly peptide phosphorylation. Philos Trans R Soc Lond B Biol Sci. 1983 Jul 05;302(1108):27-32. [PubMed: 6137004]
- 13.
-
Adams A, Redden C, Menahem South. Characterization of homo fructose-one,6-bisphosphatase in control and deficient tissues. J Inherit Metab Dis. 1990;xiii(half dozen):829-48. [PubMed: 1964188]
- 14.
-
van den Berghe G. Disorders of gluconeogenesis. J Inherit Metab Dis. 1996;xix(4):470-vii. [PubMed: 8884571]
- xv.
-
Raza Thou, Arif F, Giyanwani PR, Azizullah South, Kumari South. Dietary Therapy for Von Gierke'south Disease: A Example Report. Cureus. 2017 Aug 08;9(8):e1548. [PMC costless article: PMC5630462] [PubMed: 29018645]
- sixteen.
-
Kishnani PS, Austin SL, Abdenur JE, Arn P, Bali DS, Boney A, Chung WK, Dagli AI, Dale D, Koeberl D, Somers MJ, Wechsler SB, Weinstein DA, Wolfsdorf JI, Watson MS., American College of Medical Genetics and Genomics. Diagnosis and management of glycogen storage affliction type I: a practice guideline of the American College of Medical Genetics and Genomics. Genet Med. 2014 Nov;sixteen(11):e1. [PubMed: 25356975]
- 17.
-
Derks TG, van Rijn M. Lipids in hepatic glycogen storage diseases: pathophysiology, monitoring of dietary management and hereafter directions. J Inherit Metab Dis. 2015 May;38(3):537-43. [PMC gratuitous article: PMC4432100] [PubMed: 25633903]
- 18.
-
Arnold GL, Griebel ML, Porterfield 1000, Brewster M. Pyruvate carboxylase deficiency. Study of a case and additional evidence for the "mild" phenotype. Clin Pediatr (Phila). 2001 Sep;40(ix):519-21. [PubMed: 11583052]
Source: https://www.ncbi.nlm.nih.gov/books/NBK544346/
Posted by: loafters.blogspot.com

0 Response to "Which Of The Following Statements About Gluconeogenesis In Animal Cells Is True?"
Post a Comment